About Event
Defining Novel Targets, Validating Endpoints, and Accelerating First-in-Class Therapeutics for Dry AMD & Geographic Atrophy
The 6th Dry AMD & GA Therapeutics Summit returns to tackle key challenges facing discovery and translational scientists. Built with the industry’s specific needs in mind, 2025's agenda spanned discovery to early clinical content. This was your one stop shop to advance and accelerate the development of safe, effective and accessible dry AMD & GA therapeutics by uncovering cutting-edge advances.
Robust new content for 2025 included overcoming the major preclinical challenges including new preclinical models recapitulating retinal microenvironment, validating new drug targets beyond complement and inflammasome systems, identifying novel imaging biomarkers, and regulatory enabled clinical endpoints for faster approvals!
Why Join Us This Year:
Discuss funding strategies with healthcare investors from PENG Life Science Ventures and ExSight Ventures, and independent strategists to learn what data, milestones, and positioning matter most in today’s competitive development landscape

Get updates on the latest clinical progress and development strategies from biopharma leaders including Apellis, Ocugen, and Clearside Biomedical to gain practical takeaways to refine your own developments and anticipate where the field is moving next

Define global regulatory expectations and learn how to de-risk early-stage programs by aligning study design, endpoints, and imaging strategies with FDA and EMA feedback, guided by regulatory consultants with proven approval experience in dry AMD

Explore novel mechanisms beyond complement including ferroptosis, mitochondrial rescue, and RPE regeneration, with insights from VisgenX and PulseSight Therapeutics to identify differentiated therapeutic approaches and evaluate which next-gen strategies are gaining traction in the clinic

Integrate AI, imaging biomarkers, and functional endpoints into your early-phase trial planning with insights from leading academics and members of the International Retinal Imaging Society to increase efficiency and align with evolving regulatory standards
Who Will You Meet?
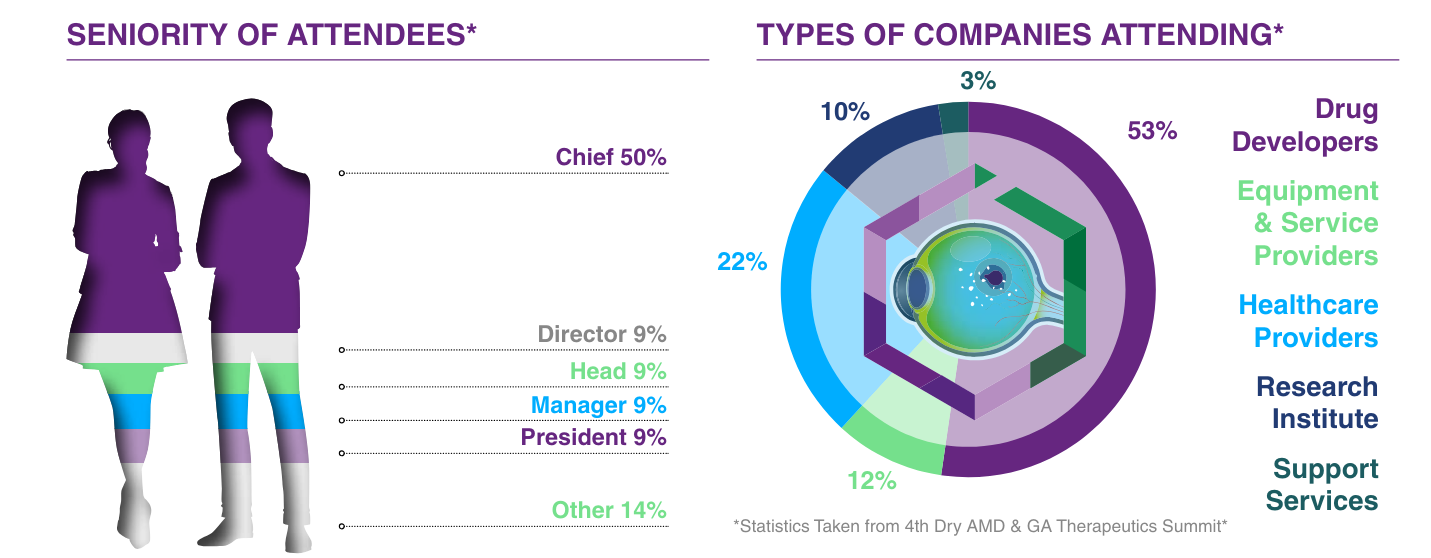
The 6th Dry AMD & Geographic Atrophy Therapeutics Summit brings together a global community of 40+ experts in clinical development, translational research, regulatory, and business development professionals from biotech, pharma, and academia advancing novel therapies for dry AMD and Geographic Atrophy.
Connect with senior biopharma leaders from Apellis, Boehringer Ingelheim, AbbVie, Genentech, Sanofi, Novartis, Aviceda, ONL Therapeutics
What Your Peers Have to Say:
“This was my first Hanson-Wade conference/meeting. I did not know what to expect, and my expectations were exceeded. Particularly, I was surprised at how forthcoming speakers were with sharing their company's data and collaboration.”
Director - Chemistry, Manufacturing & Controls, Gene & Cell Therapy & Early Discovery, Rejuvitas
“The meeting presented a great opportunity for academics to interact with pharma and see where we really stand on getting drugs to patients. I think there was a lot of great discussions.”
Assistant Professor, Johns Hopkins University
"Focused meeting with willingness of participants to share ideas and experience."
Chief Scientific Officer, Akari Therapeutics
“Excellent knowledgeable speakers, interactive, wide range of industry from start-ups to strategies, discussion of challenges not just successes including regulatory perspectives. The basic scientists and clinicians added great flavor.”
Associate Director, Ophthalmology, Dompe
“The almost complete coverage of assets under development and the forthrightness of the presenting speakers.”
Founder & Chief Executive Officer, Drusolv Therapeutics
